Covid Clinical Trial Mobile App
Integrating electronic consent and voice data recording to enhance patient-reported outcome studies.

Objective:
Design a mobile application for a fully decentralized clinical trial that supports electronic consent and includes features for voice data recording, symptom tracking, and swab documentation.
Context and Challenge:
Originally initiated to explore correlations between voice data and RSV, the project scope expanded to include SARS-CoV-2 amidst the Covid-19 pandemic. This shift brought about significant changes in study protocols and vendor relationships, requiring extensive collaboration and rapid adaptation to evolving requirements.
My Role:
As the lead designer, I was responsible for:
Collaborating closely with operations, project management, and engineering teams.
Creating and refining diagrams, user flows, and information architecture to visualize potential study workflows and accommodate changes in study protocol.
Designing, presenting, and iterating on high-fidelity screens with the client
Initial Planning:
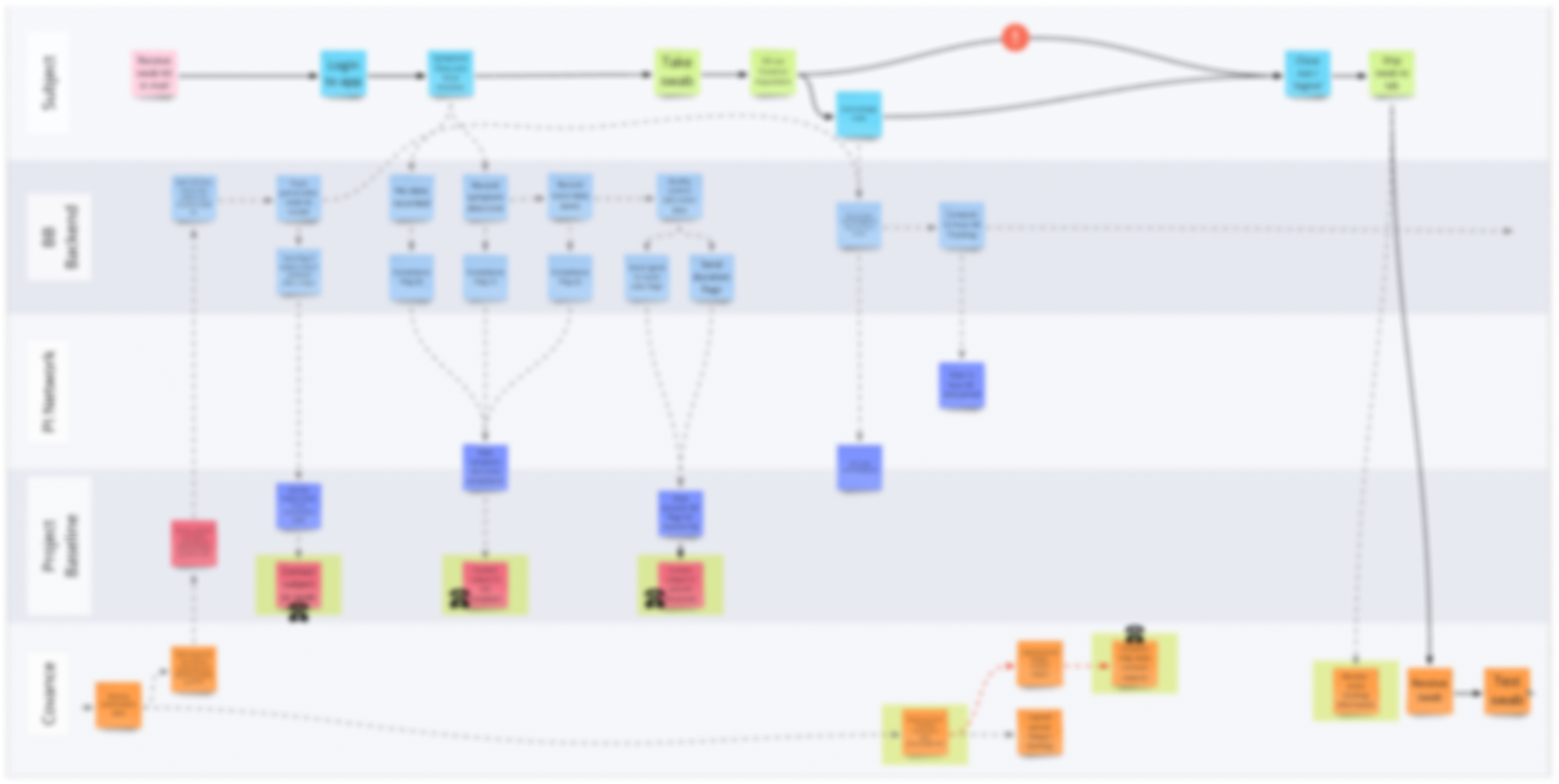
Working closely with a representative from the client, I created many diagrams to visualize the potential structures of the study. With fluctuating vendors and study requirements, these visualizations proved vital for ensuring agreement on the design of the study.
Initial high-level flow of the entire study.
Example swimlane process diagram highlighting hand-offs in the study. (Blurred for confidentiality)
Development of Key Features:
Once the final vendors were confirmed and the protocol was solidified, we began updating the application to accommodate the finalized study. This project introduced several challenging aspects we hadn't encountered before, including recording swabs, monitoring adverse events, and implementing eConsent for remote app downloads within the application. I developed workflows and collaborated closely with engineers, the client, and management to devise solutions. Once these workflows were internally agreed upon, I presented them to obtain full sign-off.
Example diagram depicting how swab results affect participant’s study cohort placement. (Blurred for confidentiality)
High-Fidelity Design:
To expedite the creation of high-fidelity screens, we leveraged a previously established style guide along with initial conceptual designs. The design team incorporated multimedia elements such as videos, GIFs, and images to create an engaging user interface, while carefully balancing complex legal requirements.
User Testing and Iteration
Post-design, I utilized Amazon’s Mechanical Turk to recruit participants for beta testing:
Conducted surveys to gather feedback after participants registered and engaged with the app’s functionalities.
Ensured the quality of voice data collection across various devices through participant feedback.
Identified and addressed pain points, significantly enhancing user experience.
Outcome and Impact
The application was successfully launched in app stores, marking the commencement of the clinical trial. The trial has since been completed and the findings are publicly accessible on ClinicalTrials.gov. This study stands as one of the largest decentralized clinical trials to date, with over 10,000 participants recruited.
This project highlighted the importance of adaptability in design, especially in a rapidly changing healthcare environment. It also underscored the value of user testing in refining digital tools that must meet stringent regulatory standards while providing a seamless user experience.
Reflections and Learnings
Conclusion
This case study not only showcases my skills in managing and executing complex design projects but also highlights my ability to innovate and adapt in response to unforeseen challenges.